Diabetes is one of America’s biggest healthcare challenges. According to the latest figures from the Centers for Disease Control, 30.4 million Americans have diabetes and an additional 1.5 million Americans get diagnosed with the disease every year.
The widespread prevalence of diabetes makes it one of the biggest markets for global drugmakers. According to a study financed by diabetes-drug maker Novo Nordisk, spending on diabetes treatment will increase from $408 billion to $622 billion between 2015 and 2030.
If the sheer size of this massive addressable market has you interested in investing in diabetes companies, here’s what you need to know about the disease, treatment options, and the companies most likely to benefit from growing demand.

What is diabetes?
Diabetes is a disease characterized by the inability to produce or respond to insulin, a hormone produced in the pancreas that regulates the amount of glucose in the blood.
A component of many carbohydrates, glucose is a simple sugar that serves as an important source of energy. In fact, it’s the primary source of energy for the brain.
In healthy people, when there are higher levels of glucose in the bloodstream, the pancreas increases insulin production to boost the uptake of glucose by the liver and muscles, where it can be stored as a future source of energy.
In diabetics, the inability to produce insulin interferes with the storage of glucose, increasing glucose levels in the blood and urine.
There are two forms of diabetes: type 1 and type 2.
Type 1 diabetes usually appears in childhood or adolescence, formerly known as juvenile diabetes. In type 1 diabetes, the pancreas produces too little or no insulin, which forces the body to produce energy from other sources. It’s not known precisely what causes type 1 diabetes, but it’s thought that genetics and viruses play a role in causing a patient’s immune system to attack and destroy the cells in the pancreas that produce insulin.
Typically developing later in life, type 2 diabetes is characterized by the body no longer responding well to the insulin produced by the pancreas, something that’s called insulin resistance. Genes and lifestyle are common causes of type 2 diabetes, with people who smoke or who are overweight being at the greatest risk of developing it.
Of the two types of diabetes, type 2 diabetes is most common. Out of the more than 30 million people with diabetes in the United States, only 1.5 million people are type 1 diabetics.

What are the risks of diabetes?
Too much glucose in the bloodstream can damage blood vessels that sustain nerves and as a result, diabetes can cause nerve damage that causes tingling, numbness, burning, or pain. Over time, this nerve damage can lead to the loss of feeling in limbs and digestive problems.
Diabetes can also damage blood cells in the kidneys that filter blood, which can cause kidney failure, and it can cause cardiovascular disease that results in heart attack or stroke. It may also increase the risk of Alzheimer’s disease.
In 2015, it was mentioned as a cause of death on a total of 252,806 death certificates, and overall, diabetes is the 7th biggest cause of death in the United States.
The soaring cost of diabetes
The amount of money spent directly on diabetes is staggering. An Institute for Alternative Futures (IAF) study funded by Novo Nordisk claims diabetes costs over $400 billion. It also estimates that the number of diabetics will increase by 54% to nearly 55 million Americans in 2030, resulting in spending on diabetes eclipsing $600 billion.
The increase in the absolute number of diabetics will drive a significant proportion of the increase in spending. However, longer-living patients will also increase spending.
Between 2000 and 2010, there was a 40% decline in deaths caused by diabetes in the U.S., and the IAF estimates we’ll see an additional decrease of 25% between 2010 to 2030. The big reason for this reduction is a drop-off in cardiovascular deaths caused by diabetes, a development that’s owed to advances in treatment that have improved the control of glucose levels, blood pressure, and cholesterol in diabetics.
New diabetes drugs are helping patients control their blood sugar levels better. However, these treatments are more expensive than prior-generation drugs. As a result, diabetes costs grew 41% between 2007 and 2012, and the costs associated with treating patients with prediabetes grew 74% between 2007 and 2012 to $44 billion, according to the IAF study.
Commonly used diabetes medications
There are many different diabetes drugs that are used to help reduce glucose in the blood, and each works a bit differently.
For example, there are five types of insulin that patients may take:
- Rapid-acting insulin, which begins working within a few minutes and can last two to four hours.
- Regular/short-acting insulin, which begins working in 30 minutes and lasts three to six hours.
- Intermediate-acting insulin, which takes effect in one to two hours and lasts up to 18 hours.
- Long-acting insulin, which takes effect in one to two hours and lasts over 24 hours.
- Ultra long-acting insulin, which begins working in one to two hours and can last 42 hours.
But insulin alone may not be enough to control glucose levels. Combination therapy regimens may also be used to help regulate blood glucose levels. These drugs include:
- Sulfonylureas, which stimulate the pancreas to release more insulin.
- Biguanides, including metformin, which lower blood glucose levels by decreasing glucose production in the liver.
- DPP-4 inhibitors, which prevent the breakdown of human glucagon-like peptide-1 (GLP-1), an enzyme that naturally lowers blood glucose.
- SGLT2 inhibitors, which keep sodium-glucose cotransporter 2 (SGLT2) in the kidney from reabsorbing glucose.
The major players
Many drugmakers market these diabetes drugs, however, Novo Nordisk’s, Eli Lilly’s, and Sanofi’s drugs are among the most widely used treatments. Each generates billions of dollars per year in sales from diabetes drugs, including insulin. The table below shows their 2017 annual results.

Data sources: Yahoo! Finance and company annual reports. Note: 1. Novo Nordisk’s results reflect exchange rates on May 17, 2018. 2. Eli Lilly’s net income figure is adjusted net income. 3. Sanofi’s net income is business net income, a non-GAAP adjusted figure and results reflect exchange rates on May 17, 2018. YOY = year over year.
Johnson & Johnson (NYSE: JNJ) is also a big player in diabetes. It markets everything from glucose meters used to measure blood glucose levels, to insulin pumps that deliver insulin directly to the pancreas, to novel medicines, including Invokana, as well as a SGLT2 inhibitor that helps the body remove more glucose from the bloodstream in urine. However, J&J’s Invokana sales have been falling because of new competition, and last year, the company said it is exploring the sale of its diabetes medical device business.
What does Novo Nordisk do?
Novo Nordisk’s diabetes market share was 27% in 2017, which makes it the biggest diabetes-drug maker in terms of market value.
The company’s top-selling diabetes medicine is NovoLog, a rapid-acting insulin that’s delivered by injection using an all-in-one, pen-sized device. However, growing demand for next-generation insulin, including the long-acting Tresiba, is what’s really moving the needle for Novo Nordisk.
Tresiba is a once-daily, new-generation insulin for type 1 and type 2 patients. Its 25-hour half-life and duration of action of at least 42 hours provide patients with greater dosing flexibility, removing the need to take insulin at the same time every day.
In 2017, growing use of Tresiba contributed to Novo Nordisk’s next-generation insulin sales growing 94% and its total sales and operating profit growing 2% and 5%, respectively. It also resulted in Novo Nordisk having a 45% share of the modern insulin market.
Victoza is another important Novo Nordisk diabetes drug. It’s a once-daily GLP-1 treatment for type 2 diabetes that won Food and Drug Administration (FDA) approval in 2010. Already a top seller, the FDA updated Victoza’s prescribing information in August 2017 to include data showing it reduces major cardiovascular events, including heart attack and stroke, in type 2 patients. That change helped Victoza’s sales improve 16% in 2017.
In the future, Novo Nordisk’s research and development spending should produce a steady stream of new drugs that work better. For example, the company recently secured approvals for Ozempic, a once-weekly, injectable GLP-1 treatment for adults with type 2 diabetes, and Fiasp, a new fast-acting mealtime insulin.
Further back in its pipeline, the company is working on game-changing medicines that could help insulate Novo Nordisk’s market share as patents on key drugs expire. NovoLog’s patent protection has already ended and patents on Tresiba and Victoza expire in 2029 and 2022, respectively.
The development of oral diabetes drugs is particularly revolutionary because insulins and GLP-1 medicines are biologics that, in their current form, would be destroyed by digestive enzymes if they were taken orally. However, Novo Nordisk’s making progress overcoming that obstacle. It’s already studying an oral formulation of Ozempic in late-stage studies and researching ways to deliver insulin via a tablet, too. A potential cure for type 1 diabetes that involves transforming stem cells into the beta cells that produce insulin is exciting, as well. Management hopes to be in a position where it can begin human trials of such an approach in a few years.
What does Eli Lilly do?
Eli Lilly gets about 44% of its revenue from diabetes drugs. In 2017, rising sales of Trulicity, Basalgar, and Jardiance resulted in over $10 billion in diabetes drug sales.
Eli Lilly’s top seller is the rapid-acting insulin , Humalog, which competes against Novo Nordisk’s NovoLog. Last year, Humalog racked up nearly $2.9 billion in sales.
The company’s fastest-growing diabetes drug is Basalgar, though. Basalgar is a biosimilar to Sanofi’s long-acting insulin Lantus, a drug that was generating over $7 billion in sales before losing its patent protection. Biosimilars are highly similar to existing FDA-approved therapies. Lantus, a biologic produced in a living organism, is impossible to precisely duplicate, but Basalgar is similar enough in design and efficacy to be used as a replacement now that Lantus’ patent has expired.
Launched in 2016, Basalgar’s been chipping away at Lantus’ market share ever since. In 2017, Basalgar’s sales surged 402% to $432 million.
Sales are also growing for Jardiance, an SGLT2 inhibitor that competes against J&J’s Invokana, and Trulicity, a once-weekly GLP-1 drug that competes against Novo’s Victoza and Ozempic. Jardiance and Trulicity revenue increased 122% to $447.5 million and 119% to $2.03 billion, respectively, in 2017.
Like Novo, Eli Lilly is committed to researching better diabetes treatments. It has two new diabetes drugs in phase 3 trials and another two new drugs that are in phase 2 trials. In addition to those drugs, it also recently launched an early-stage trial for a closed-loop, automated insulin delivery system. If successful, this system could improve glucose control and reduce patient burden by connecting an insulin pump to a continuous glucose monitor (CGM), an approach that’s quickly gaining traction (a bit more on that in a minute!).
Overall, Eli Lilly’s got plenty in the works, and that’s critical given that Humalog’s already lost its patent protection. The FDA approved Sanofi’s Humalog biosimilar, Admelog, in December 2017. Patents on Jardiance and Trulicity don’t expire until 2025 and 2024, respectively (not including possible patent extensions), so they’ll remain key contributors to sales for a while.

What does Sanofi do?
At Sanofi, diabetes revenue is falling by double-digit percentages because of Lantus’ expiring patent. For instance, diabetes sales dropped 15.7% in Q1 2018 because of increased competition.
Nevertheless, the company still generates over $4 billion in annualized diabetes revenue, but it’s not resting on its laurels.
As I mentioned above, the FDA recently approved its Humalog biosimilar, Admelog, and it became available in the U.S. in April 2018. Sanofi’s also awaiting an FDA decision on sotagliflozin in 2018, a drug it licensed from Lexicon Pharmaceuticals (NASDAQ: LXRX) in 2015 for $300 million up front, up to $430 million in development and regulatory milestones, up to $990 million in specified sales milestones, and tiered, escalating royalties ranging from low double-digit percentages to 40% of net sales.
If sotagliflozin wins approval, it will become the first drug available that controls blood sugar levels by inhibiting both SGLT2 and SGLT1, which is responsible for reabsorbing glucose in the gastrointestinal tract. However, an approval for sotagliflozin isn’t guaranteed given trial results last year that led many to question its safety. Specifically, the trial showed an increased rate of diabetic ketoacidosis (DKA), a life-threatening buildup of acids in the blood that occurs when blood sugar is too high for too long.
The safety concerns shouldn’t be ignored, but they may not cause the FDA to sideline sotagliflozin. That’s because SGLT2 drugs are already associated with a doubling in the risk of DKA, yet they’re still widely used.
Sanofi’s also conducting phase 3 studies on SAR341402, a rapid-acting insulin that could challenge NovoLog, and efpeglenatide, a long-acting GLP-1 drug that could compete against Trulicity and Ozempic. The pipeline at Sanofi could allow it to regain some of the momentum that’s been lost since Lantus’ patent protection ended.
What’s next in diabetes treatment
The market for diabetes medicines is massive, but there’s also a big opportunity associated with developing next-generation devices and digital solutions that improve patient outcomes, too.
For instance, there’s significant innovation occurring in how diabetics track their blood sugar and dose their insulin. In the past, patients relied on finger sticks and glucose meters to measure blood glucose levels to determine when to take insulin injections. However, that approach is less than ideal because studies have shown that diabetics spend roughly 70% of their day outside their desired blood glucose range.
To increase the amount of time spent within a healthy blood sugar range, companies are developing medical devices that continuously monitor blood glucose levels and insulin pumps that deliver insulin more regularly.
In April 2017, Medtronic (NYSE: MDT), a large medical device maker, launched the MiniMed670G, a first-generation system that automates glucose monitoring and insulin delivery for type 1 diabetes patients. It pairs a proprietary monitor that measures blood glucose every five minutes with a pump to deliver insulin as needed. In trials, the MiniMed670G significantly improved blood glucose levels and reduced the risk of hypoglycemia, a condition in which blood glucose falls to dangerously low levels.
As of December 2017, the MiniMed670G is only being used by 20,000 patients, but use of these automated systems could increase substantially if systems being evaluated by other companies — including Eli Lilly — pan out.
Systems from Insulet (NASDAQ: PODD) and Tandem Diabetes Care (NASDAQ: TNDM) are among those closest to competing against Medtronic. In Insulet’s case, it’s pairing up its popular OmniPod, a device that can be attached to the skin to deliver insulin for up to three days, with Dexcom’s (NASDAQ: DXCM) popular G5 CGM, which charts blood glucose levels over time to provide greater insight. Tandem’s combining its t:slim insulin pump with Dexcom’s G5 CGM, too.
Dexcom won FDA approval of its latest monitor, the G6, in March, and Insulet and Tandem have already said they plan to incorporate it into their automated systems in the future. The G6 is a significant advance for patients because its quarter-sized sensors can be worn for 10 days at a time and it eliminates the need for finger sticks, which were previously required to calibrate DexCom’s CGMs. The G6 is also the first CGM to secure approval for use on its own or alongside other devices, including pumps, insulin injectors, and mobile apps.
Dexcom is working with Verily, Alphabet’s healthcare start-up, to create even smaller, longer-lasting sensors that can be worn on the body, but it’s not the only company hoping to use technology in diabetes. Apple Inc. is reportedly working on technology that incorporates sensors into wearables for real-time insight and announced a big commitment to healthcare apps that can turn personal data into actionable healthcare solutions.
Are diabetes stocks good buys?
The IAF estimates that one-third of U.S. adults have prediabetes, and that means that over 84 million Americans are at risk of developing the disease and requiring treatment.
An increasingly larger addressable patient population suggests that owning diabetes stocks could be smart. Income investors might want to consider Novo Nordisk, Eli Lilly, or Sanofi because they’re all mature biopharma companies with solid dividend track records.
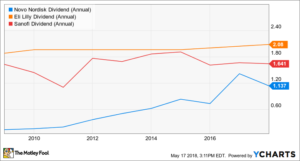
A word of caution, though: Any number of things could go wrong or change in the marketplace that could cause investors in these companies to lose money. The market is highly competitive and battling for market share could dent these companies’ profitability if it results in a price war. There’s also the risk that new diabetes treatments displace existing treatments or that clinical trials of promising diabetes therapies fail. It’s important to remember that all of these companies market other drugs that are unrelated to diabetes, so they’re not pure plays in the diabetes indication.
Smaller medtech stocks — including Insulet, Tandem, and Dexcom — get all their revenue from diabetes, so they’re the stocks to buy if you’re hunting for a pure play, but their dependence solely on diabetes for revenue also means that they’re the riskiest stocks to include in your portfolio.


